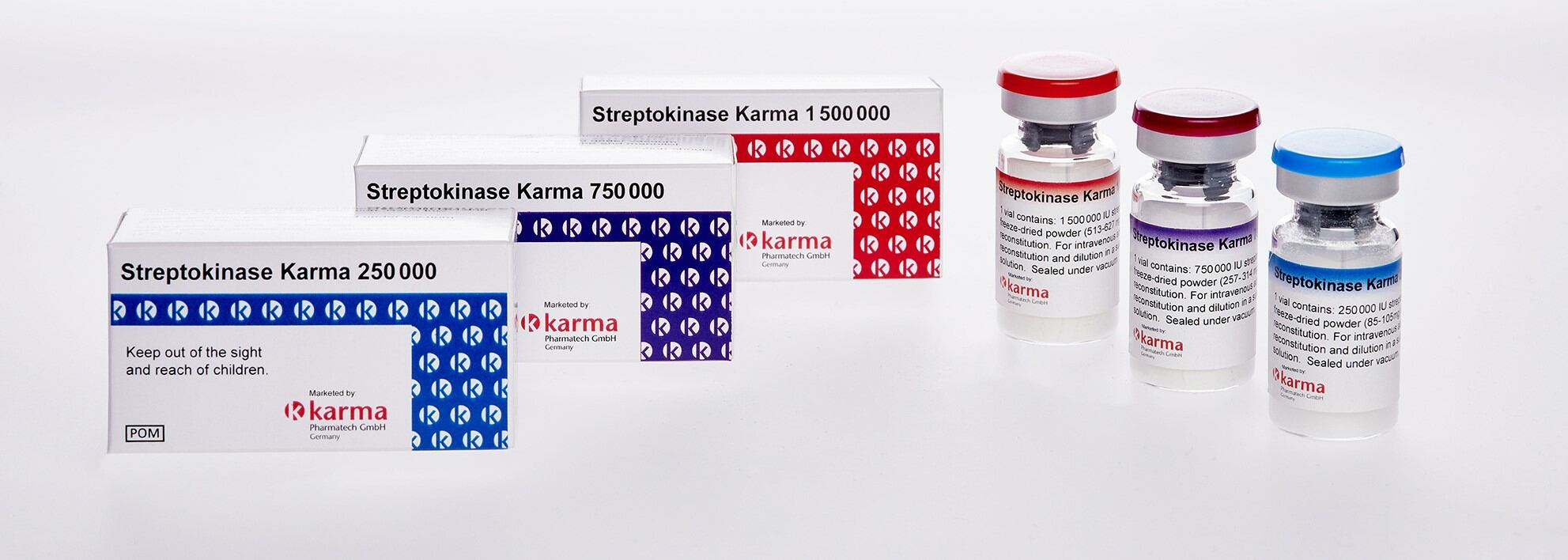

Streptokinase was discovered in 1933 and is a protein extracted from certain strains of beta hemolytic streptococcus. It activates the fibrinolytic system: First it binds onto plasminogen to build an activator complex, which then converts plasminogen into the fibrinolytic enzyme plasmin. Plasmin is an enzyme that is able to dissolve the fibrin cloth and is therefore used in the thrombolytic threapy.
Streptokinase is indicated for the treatment of acute myocardial infarction, pulmonary embolism and deep vein thrombosis. Streptokinase is one of the most cost effective thrombolytic drugs, and has been listed once again on the 2019 WHO Model List of Essential Medicines (EML). This list contains the medications considered to be most effective and safe to meet the most important needs in a health system. The list is frequently used by countries to help develop their own local lists of essential medicine.
- Widely tested in mega trials
- Manufactured in Germany
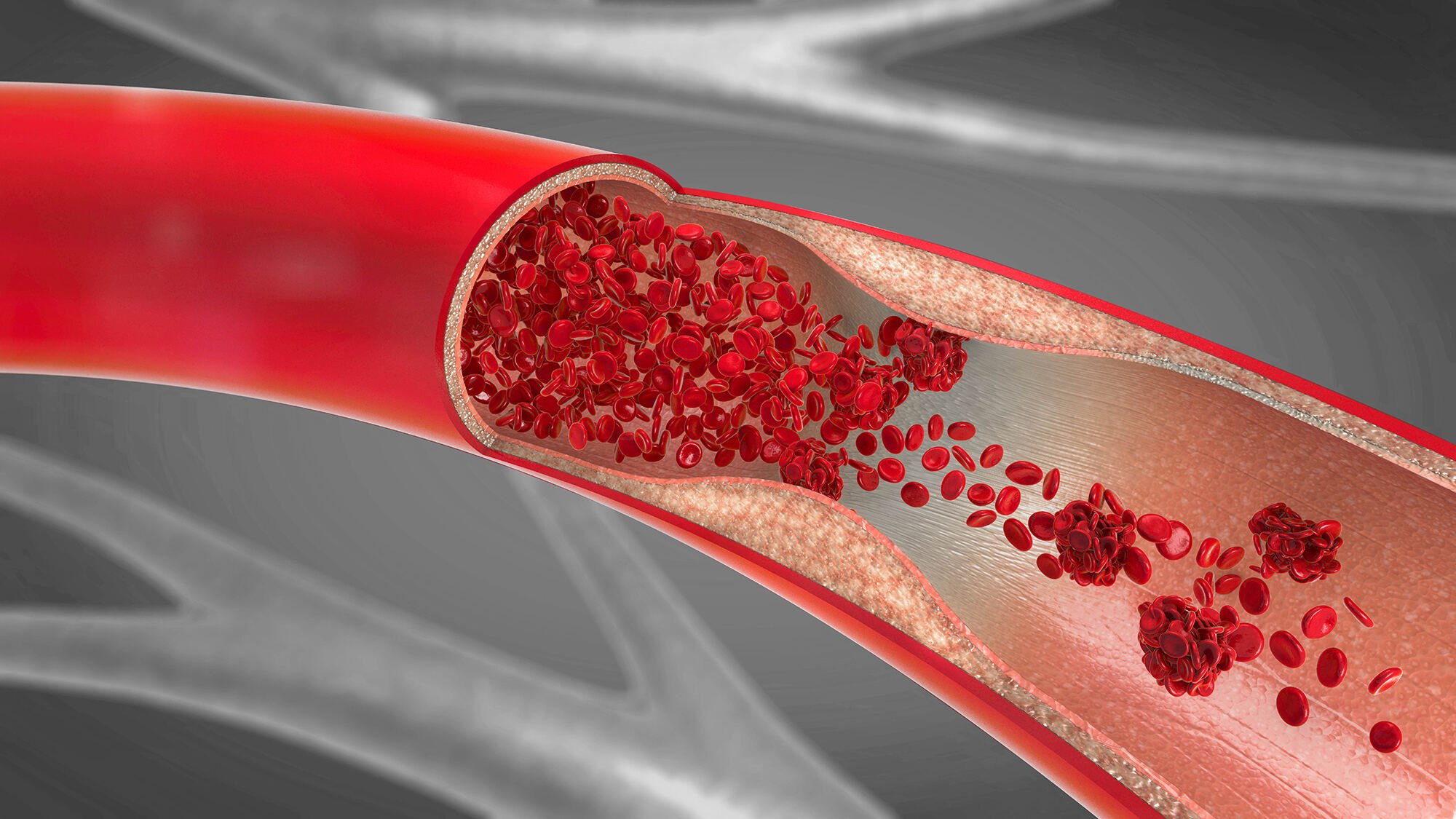
Streptokinase Mode of Action
Streptokinase activates the fibrinolytic system indirectly in a reaction of two different phases:
In the first phase, streptokinase binds onto plasminogen to form an activator, the Plasminogen-Streptokinase activator complex (Plg-SK activator complex).
In the second phase, the activator complex converts plasminogen into the fibrinolytic enzyme plasmin. Plasmin is able to dissolve the fibrin matrix involved in the thrombus structure with subsequent thrombolysis.
Kinetic investigations have shown that the thrombolytic potential of streptokinase or the activator complex depends on the amount of drug used.

Clinical Trials overview
The efficacy and safety of streptokinase in the treatment of acute myocardial infarction was most widely tested in clinical trials which included more than 91.000 patients.
GISSI Study
ISIS-2 Study
GISSI-2 International Study
ISIS-3 Study
GISSI Study
The first Italian GISSI Study investigated the efficacy and safety of streptokinase in the treatment of acute myocardial infarction. 11806 patients with AMI were randomized to receive either streptokinase (1.5 million IU in 1 hour) intravenously or routine coronary care with 12 hours after onset of symptoms.
Although the greatest benefit was achieved in those patients treated within the first hour of onset of symptoms (mortality rate of the streptokinase group was 8,2% vs. 15,4%for the control), the mortality reduction remained significant even in those patients treated between 3 and 6 hours after the onset of chest pain (mortality rate of the streptokinase group was 11,7% vs. 14,1% for the control group).
In addition, the incidence of adverse reactions associated with the high-dose, short-term lysis with streptokinase, particularly major bleeds, anaphylactic reactions and stroke were very low.

ISIS-2 Study
This study investigated the efficacy and safety of i.v. streptokinase (1,5 Mio IU in 1 h), oral aspirin (160 mg/day for one month) or both were investigated in AMI patients. 17187 patients with suspected AMI were randomized to receive either streptokinase, aspirin, both drugs or neither within 24 hours from onset of symptoms.
Streptokinase alone and aspirin alone each produced a highly significant reduction of infarct mortality. 9,2% for streptokinase vs. 12 % for the control group (23,3 % reduction of mortality) and 9,4 % for aspirin vs. 11,8% for the control group (20,3 % reduction of mortality).
The combination of intravenous streptokinase and oral aspirin was significantly better than either drug alone: 8,0% for the combination vs. 13,2% for the control group (39,4 % reduction of mortality).
In terms of safety, streptokinase was associated with 3,5% of minor bleeds, while the incidence of major bleeds was very low (0,5%). Cerebral haemorrhage was reported in only 0,1% for the streptokinase group. Reports of allergic reactions were in most cases confined to shivering, pyrexia or rashes; no reports of anaphylactic shock were confirmed.

GISSI-2 International Study
In a second GISSI trial (GISSI 2 International) , the efficacy and safety of two thrombolytic agents (streptokinase and tPA) with or without heparin were investigated in Acute myocardial infarction.
20.891 patients with suspected AMI were randomized to receive either 1,5 million IU of streptokinase in 30-60 minutes or 100 mg of tPA (alteplase) over 3 h within 6 hours from onset of symptoms. The patients were also randomized to receive subcutaneous heparin (12.500 IU twice daily until discharge) beginning 12 hours after the start of thrombolytic therapy. All patients without specific contraindications were given aspirin orally (300-325 mg / day).
In terms of efficacy, no significant differences in mortality were found between streptokinase (8,5%) and tPA (8,9%) or between thrombolysis with heparin (8,5%) and thrombolysis alone (8,9%).
Furthermore, the incidence of major cardiac complications was comparable in the different groups. However, stroke was significantly higher in the tPA group than in the streptokinase group (1,3% v. 1%). More major bleeds occurred with streptokinase than with tPA (0,9% vs. 0,6%). Thrombolysis with heparin was likewise associated with significantly higher rate of major bleeds than thrombolysis alsone (1,0% versus 0,5%).

ISIS-3 Study
In a third ISIS trial three thrombolytic agents with or without heparin anticoagulation were compared. 41.299 patients with suspected AMI were randomized to receive either streptokinase (1,5 mio IU over 1 hour), tPA (duteplase 0,6 MU/kg over 4 h) or APSAC (anistreplase 30 U over 3 min) within 24 hours from onset of symptoms. All patients were to receive oral aspirin (162 mg /day). In addition, half of the patients were randomly allocated SC heparin (12.500 IU twice daily) for 7 days starting 4 hours after thrombolysis.
In terms of efficacy, significant difference in mortality could neither be detected between streptokinase (10,6%), tPA (10,3%) or APSAC (10,5%) not between thrombolysis with heparin (10,3%) or thrombolysis without heparin (10,6%).
The most dramatic complication, i.e. haemorrhagic stroke, was significantly higher in the tPA (0,66%) and APSAC (0,55%) than in the streptokinase group (0,24%). The incidence of major bleeds requiring blood transfusion was quite similar in the three treatment groups (streptokinase 0,9%, tPA 0,8% and APSAC 1%).
On the other hand, the incidences of reinfarction, allergic reactions with persistent symptoms and hyptotension requiring drug treatment were significantly lower in the tPA group than in the streptokinase group and in the APSAC group.
Thrombolysis with heparin produced significantly higher incidence of bleeding complications and haemorrhagic stroke than thrombolysis alone.
 Downloads
Downloads